
– A Flow Cytometry-Based Blood Test to Guide Cancer Management

NeutroFlow is an EIC Transition funded project aiming to develop and validate a point-of-care, flow cytometry-based diagnostic test that detects a novel cellular biomarker in the blood for predicting response to immune checkpoint inhibitor (ICI) therapy for cancer. The test will enable informed treatment decisions, advancing personalized medicine for cancer patients.

Clinical Workflow of the NeutroFlow Blood Test
Pretreatment blood sample

Biomarker detection by flow cytometry

Patient report

Personalized treatment plan
Current Challenges in ICI Treatment
>50% of cancer patients are eligible for ICIs, but only ~14% respond to these treatments, with outcomes discovered after 3-6 months.
There is a risk of serious side effects known as immune-related adverse events.
Treatment costs are high.
Current biomarker tests for guiding treatment decisions are not optimal, and usually require an invasive tumor biopsy.
There is an unmet need for novel biomarkers with superior predictive performance. Ideally, such biomarkers would be minimally invasive (e.g., measured in a blood sample).
Powerful predictive biomarkers would expand the benefit of ICIs to more patients, improve therapy outcome and quality of life, avoid the risk of irAEs in ineligible patients and reduce economic burden.




The NeutroFlow test is designed as a simple-to-use, rapid, flow cytometry-based test for measuring the Ly6Ehi neutrophil biomarker in blood samples.
The test will require a standard blood draw before treatment begins, and is therefore minimally invasive, convenient, and compatible with clinical workflow. The blood will be analyzed at the point-of-care using standard hospital equipment, saving time and lowering costs.




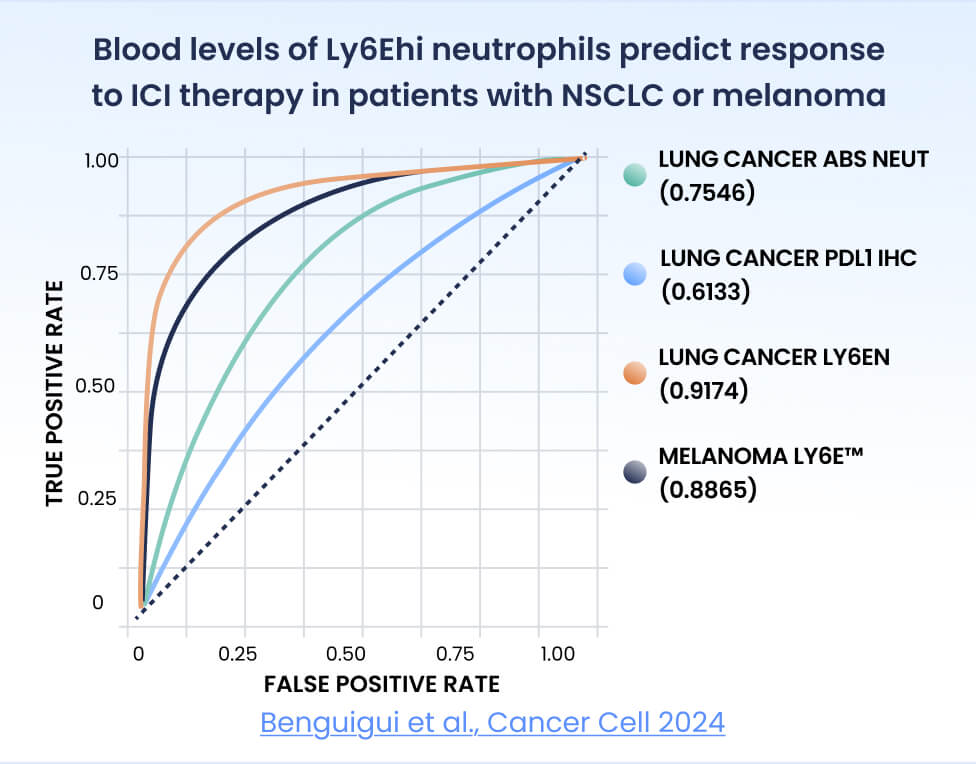
The Ly6Ehi neutrophil biomarker has shown superior predictive performance compared to standard biomarkers. It thus shows potential for development into a highly accurate clinical test for guiding treatment decisions:
(i) The Neutroflow test would focus the application of ICIs more effectively on likely responsive patients.
(ii) Offering available alternatives to patients who are not likely to benefit from ICIs would avoid potential ICI-related toxicities and reduce economic burden.
(iii) As a potential pan-cancer biomarker test relevant for multiple solid tumor types, it will potentially expand the benefit of ICIs to more patients.


The NeutroFlow project is funded by the European Innovation Council (EIC) Transition programme under Horizon Europe – Grant Agreement Number 101213660


